

Hydrogen atom cannot be a center atom because hydrogen atom cannot make more than 1 bonds. HCHO molecule, Total pairs of electrons are six in their valence shells. Total electron pairs are determined by dividing the number total valence electrons by two. Pairs = σ bonds + π bonds + lone pairs at valence shells Total valence electrons = 2 + 6 + 4 = 12.valence electrons given by carbon atom = 4 * 1 = 4.valence electrons given by oxygen atom = 6 * 1 = 6.
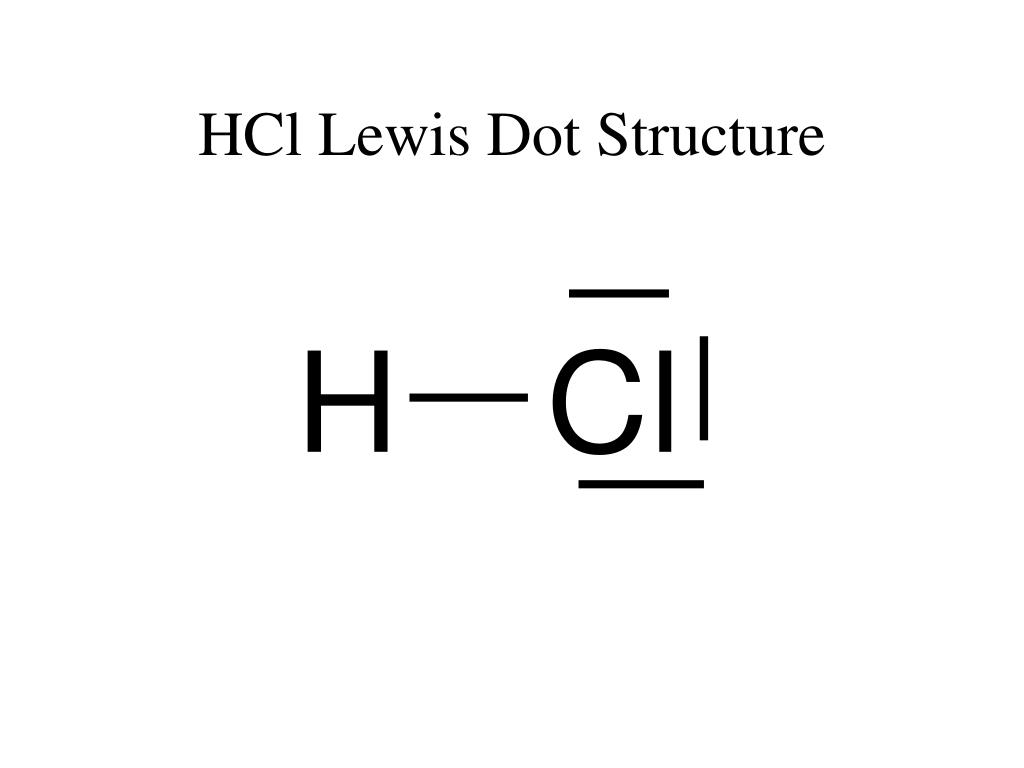
valence electrons given by hydrogen atoms = 1 * 2 = 2.Now, we know how many electrons are there in

As well, carbon is IVA group element and has fourĮlectrons in its last shell. Is a group VIA element in the periodic table and contains six electrons in its last shell. Total number of electrons of the valance shells of HCHO moleculeĮlement in the periodic table and contains only one electron in its last shell.


 0 kommentar(er)
0 kommentar(er)
